** Progress in Earth and Planetary Science is the official journal of the Japan Geoscience Union, published in collaboration with its society members.
Gallery View of PEPS Articles
Review
Solid earth sciences
202301202301
Significance of the high-pressure properties and structural evolution of gas hydrates for inferring the interior of icy bodies
Hisako Hirai, Hirokazu Kadobayashi
Methane hydrate, Hydrogen hydrate, High-pressure properties, High and low temperatures, Structural evolution, Transition mechanism, Guest orientational ordering, Molecular dissociation, Icy bodies, Diamond-anvil cell (DAC)
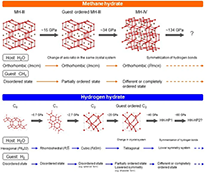
An overview of the structural evolution of (top) MH and (bottom) HH after transition to the filled-ice structure, except for HH-C0. With increasing pressure, the guests undergo stepwise orientational ordering accompanied by structural changes. Hydrogen-bond symmetrization of the host was predicted to occurs at even higher pressures.
Hydrogen, methane, and water ice are among the most abundant materials in the universe. Based on experimental, theoretical, and spacecraft data, gas hydrates consisting of gas and water ice have been predicted to exist throughout the universe. This review discusses the high-pressure properties of two common gas hydrates (methane and hydrogen hydrates) at low and high temperatures based primarily on experimental results. Gas hydrates consist of a water molecule host and a gaseous guest. They have a clathrate structure at low pressure and a filled-ice structure at high pressure. The host encloses the guest, and a specific interaction occurs between the guest and host, resulting in unique physical properties. When subjected to pressure, gas hydrates undergo various phase changes. Based on pressure and guest size, a general rule for phase changes occurring in gas hydrates exists. Analysis of the phase-transition mechanism shows that some cages are maintained after the transition to the next clathrate structure, while others are recombined into different cages of the next structure. This is a novel mechanism that can be called “cage recombination mechanism.” Low-temperature and high-pressure experiments have revealed that as the pressure increases, the guest molecules undergo a stepwise progression of orientational ordering, i.e., restriction of free rotation, which induces structural changes that stabilize the structure at high pressure. Theoretical studies have predicted that hydrogen-bond symmetrization in the host occurs at even higher pressures, further stabilizing the structure. Thus, hydrates respond to environmental changes such as pressure to achieve self-organization by the orientational ordering of the guest and hydrogen-bond symmetrization of the host. Additionally, results of high-temperature and high-pressure experiments conducted at conditions comparable to those in Neptune’s ice mantle show that methane hydrate decomposes into solid methane and ice VII, both of which melt at further elevated temperatures. Then, the methane molecules undergo further molecular dissociation to form diamonds. These findings are valuable for modeling the interiors of icy planets and understanding how magnetic fields and heat are generated.