** Progress in Earth and Planetary Science is the official journal of the Japan Geoscience Union, published in collaboration with its 51 society members.
** Progress in Earth and Planetary Science is partly financially supported by a Grant-in-Aid for Publication of Scientific Research Results to enhance dissemination of information of scientific research.
Gallery View of PEPS Articles
Review
Solid earth sciences
201404201404
Water-melt interaction in hydrous magmatic systems at high temperature and pressure
Bjorn Mysen
Hydrous magma, Aqueous fluid, Melt structure, Viscosity, Isotope partitioning, Partial melting, Water solubility, Silicate solubility, Glass transition, Solution mechanism
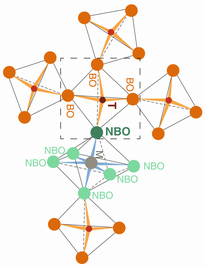
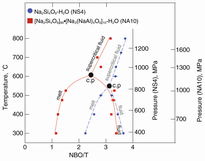
Degree of silicate polymerization, NBO/T. In silicate in hydrous melts, silicate-saturated aqueous fluid, and supercritical fluid in a hydrous Na-aluminosilicate system
Experimental data on the structure and properties of melts and fluids relevant to water-melt interaction in hydrous magmatic systems in the Earth's interior have been reviewed. Complex relationships between water solubility in melts and their bulk composition [Al/Si-ratio, metal oxide/(Al + Si) and electron properties of metal cations] explain why water solubility in felsic magmas such as those of rhyolite and andesite composition is significantly greater than the water solubility in basalt melts. The silicate solubility in aqueous fluid is also significantly dependent on composition with metal oxide/(Al + Si) and electron properties of the metal cations, the dominant variables. Hydrogen bonding is not important in hydrous fluids and melts at temperatures above 500°C to 550°C and does not, therefore, play a role in hydrous magmatic systems. The properties of hydrous melts and aqueous solutions are governed by how the silicate speciation (Qn species, where n is the number of bridging oxygen in an individual species) varies with bulk composition, silicate composition, temperature, and pressure. The reactions that describe the interactions are similar in melts, fluids, and supercritical fluids. The degree of melt polymerization caused by dissolved water varies with melt composition and total water content. Silicate- and alkali-rich felsic magmatic melts are more sensitive to water content than more mafic magmas. Transport and configurational properties of hydrous magmatic melts can be modeled with the aid of the Qn speciation variations. Liquidus and melting phase relations of hydrous systems also can be described in such terms, as can minor and trace element partition coefficients. Stable isotope fractionation (e.g., D/H) can also be rationalized in this manner. Critical to these latter observations is the high silicate concentration in aqueous fluids. These components can enhance solubility of minor and trace elements by orders of magnitude and change the speciation of H and D complexes so that their fractionation factors change quite significantly. Data from pure silicate-H2O systems cannot be employed for these purposes.