** Progress in Earth and Planetary Science is the official journal of the Japan Geoscience Union, published in collaboration with its 51 society members.
** Progress in Earth and Planetary Science is partly financially supported by a Grant-in-Aid for Publication of Scientific Research Results to enhance dissemination of information of scientific research.
Gallery View of PEPS Articles
Research
Solid earth sciences
201703201703
Hydrogen mobility in transition zone silicates
Caracas R, Panero W R
Diffusion, transition zone, Wadsleyite, Ringwoodite, Transport, Electrical conductivity, Defects, Hydrogen
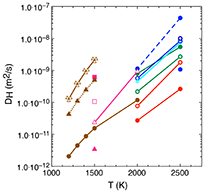
Hydrogen diffusion coefficients in ringwoodite and wadsleyite as a function of temperature. Mg↔2H, Si↔Mg+2H, and Si↔4H defect mechanisms are respectively plotted in blue, green, and red (see the caption of Figure 7 for details).
We study the hydrogen mobility in ringwoodite and wadsleyite considering multiple charge-balanced defects, including Mg < = > 2H, Si < = > Mg + 2H, and the hydrogarnet defect, Si < = > 4H, using molecular dynamics simulations based on the density functional theory at transition zone pressures and temperatures between 1500 and 2500 K. We determine the diffusion coefficients and study in detail the mechanism of hydrogen mobility during lengthy simulations. Our results show that temperature, water concentration, and defect mechanism have a significant effect on mobility. We find that the fastest diffusion is for the Mg < = > 2H defect, while H is more mobile when incorporated as Si < = > Mg + 2H than as hydrogarnet defects. The computed diffusivities for ringwoodite are larger than for wadsleyite: at 2000 K, diffusivity is 1.13 × 10−09 m2/s for ringwoodite compared to 0.93 × 10−09 m2/s for wadsleyite. In general, the hydrogen atoms spend on the order of tens of picoseconds or more trapped in or around the vacancy sites with net migration between sites over timescales of tens of femtoseconds. At 2500 K, some of these hydrogen excursions take place over several angstroms, while at 2000 K, they do not always result in net diffusion. At 1500 K, most of the defects fail to make excursions from their defect sites resulting in diffusion.